The Pressure’s On! Advanced Experiment
Background
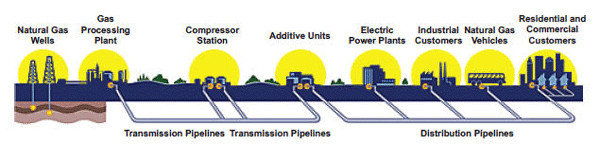
Natural gas travels from wellhead to processing plant to gas utility to customer through a series of transmission and distribution pipelines. The gas is compressed to make it move through the pipes, which range in size from 42 inches out in the field to less than an inch in diameter in your home. To maintain that pressure, compressor stations are located every 50 to 100 miles along the approximately 300,000 miles of transmission pipeline in the United States.
The natural gas delivery system is based on the principle that a gas will flow from an area of high pressure to an area of low pressure. Compressing a gas also reduces its volume, which enables distribution companies to store large amounts of natural gas for use during peak demand. In this activity you are going to investigate some of the effects of pressure on a gas.
Materials
- 1 balloon
- 1 syringe, the type with no needle (10-ml or larger; can be obtained at any pharmacy or veterinarian’s office)
- 1 large eraser
- 1 ballpoint pen
- 4 hardcover books of the same size/weight
- cooking oil (a drop)
Part I
- Blow up a balloon and pinch the neck shut. Gently squeeze the balloon with your other hand.
Does the balloon push back?___________________________________________________________Which is under greater pressure: the air inside the balloon or the air outside the balloon? __________________________________________
- Unpinch the neck of the balloon. What happens to the air inside of the balloon? Why?
Part II
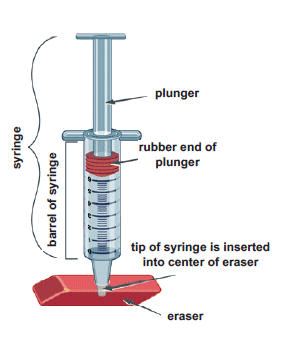
- Use a ballpoint pen to poke a hole in the center of an eraser. (Do not poke the pen all the way through the eraser.) Remove the plunger from the syringe, and then insert the tip of the syringe into the eraser.
- Rub a drop of cooking oil around the rubber end of the plunger so it will slide easily inside the barrel of the syringe. Hold the syringe upright, with the eraser flat on the table. Insert the plunger into the syringe and press down as far as you can. Then release the plunger. It should pop back up most of the way. This means no air is leaking out of the tip of the syringe.
- Note the position of the plunger in the syringe. This is the starting volume of air. Write it in the first row of the table below.
- Ask a partner to hold the syringe while you place several books on the plunger, one at a time. Observe what happens to the volume of air in the syringe. Then remove the books one by one. Record your observations in the table on the next page.
| Number of Books Pressing on Plunger | Volume of Air in Syringe (use whatever units are printed on the syringe) |
|---|---|
| 0 | |
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 3 | |
| 2 | |
| 1 | |
| 0 |
- Review your results. What is the relationship between the pressure and volume of a gas?
